UT Health Neurosurgery
As a nationally recognized medical center, we continue to experience unprecedented growth and expansion in both the clinical and academic arenas.
Academics
Our mission is to effectively educate and train the next generation of residents, fellows, students, investigators, and physician-scientists.
Research
Our goal is to maintain a rigorous, translational, and impactful research program that investigates different issues relating to neurosurgery and neurology.
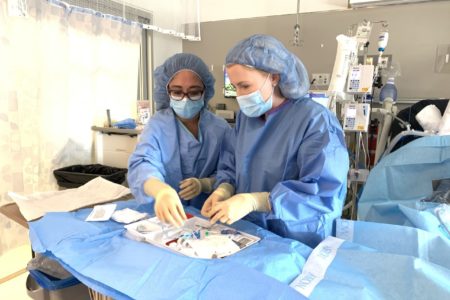
Clinical Practice
We treat conditions of the brain, skull, spine, and nerves. Our physicians have been trained in a variety of specialties, including cerebrovascular, endovascular, pediatric, and neurocritical care.

Welcome from the Chair
I love working inside the Department of Neurosurgery.
I love working with residents, researchers, faculty members, fellows, and advanced practice providers. I love teaching and patient care and getting the chance to feel like my work has made a significant impact on my community at the end of the day.
If you like that feeling too and you’re interested in what we have to offer, I welcome you to our department with open arms and encourage you to come join our family.
It’s no exaggeration to say that this department is a family.
We learn and work together. We eat together. We laugh with each other and celebrate our proudest accomplishments. We have the opportunity to grow together and help each other become better people. Better surgeons. Better scientists. Better caregivers.
I’m excited to work with you, and I know I’m not the only one! Please reach out to our staff if you have any questions about our residency program, fellowships, research efforts, or other professional opportunities.
Looking forward to future collaborations,
John R. Floyd II, MD
Chair, UT Health Department of Neurosurgery
Professor of Neurosurgery and Otolaryngology
Carl Raba Family Chair in Neuro-Oncology
Your contribution makes a difference
The assistance that we receive from donors will be used to advance the Department of Neurosurgery’s three main missions: to provide compassionate, skilled, and state of the art medical care to our community, to educate highly qualified, ethical and caring future neurosurgeons, and to advance the fields of neuroscience through the performance of groundbreaking scientific research.