Arteriovenous Malformations
What is an AVM?
There are three major types of blood vessels in the brain: arteries (which carry blood away from the heart to the cells of the brain), veins (which carry blood away from the cells of the brain back to the heart), and capillaries (which connect the arteries and veins together). Capillaries are the smallest blood vessels; they deliver oxygen and nutrients from the blood to the surrounding brain tissues and pick up waste products. A cerebral arteriovenous malformation (AVM) is an abnormal tangle of arteries and veins in the brain that do not have any capillaries connecting them together (Fig.1). Experts debate what the exact cause of AVMs might be, but many believe that people are simply born with them.
|
|
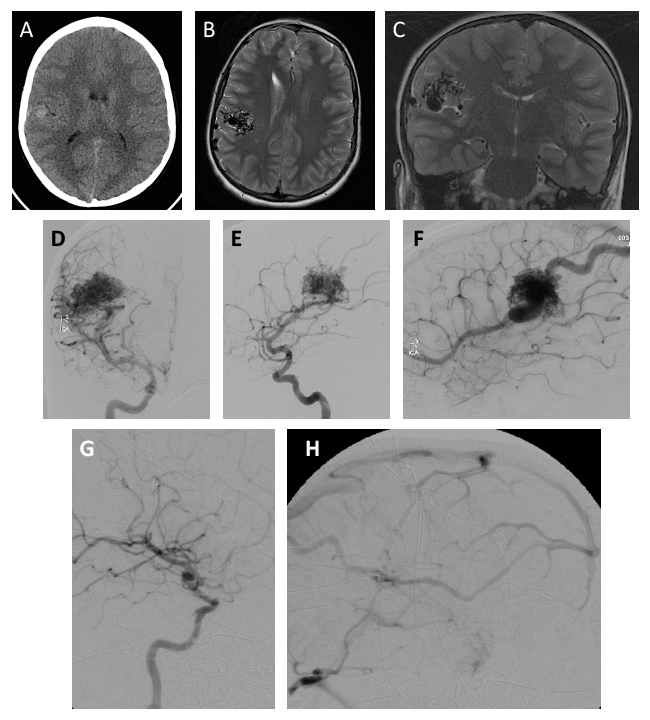
| Figure 1. (A) Computed tomography demonstrated a dense lesion in this patient’s right parietal lobe (white arrow). (B) Magnetic resonance imaging (MRI) revealed an AVM located in the parietal lobe that (C) extended into the sylvian fissure. (D–F) Digital subtracted angiograpy (DSA) revealed the location of the AVM nidus (white arrows) and venous drainage (black arrows). A craniotomy was performed and complete resection of the lesion was achieved. (G–H) Follow-up DSA demonstrated no residual contrast filling of the AVM. |
There are three structural components to an AVM: the “feeding arteries” that supply the AVM with blood, the “draining vein(s)” that take blood away from the AVM, and the cluster of abnormal blood vessels (also called the “nidus”) located between them. Under normal conditions, when a bed of capillaries is present, there is a significant difference between the pressure of the blood carried by the arteries and the pressure of the blood carried by the veins. Arterial blood comes in at a higher pressure, but much of that pressure dissipates after it travels through the capillaries and finally reaches the veins. Because AVMs have no capillaries, the high-pressure blood rushes almost directly from the feeding arteries to the draining vein(s). The veins must stretch and enlarge to accommodate the extra pressure. This process wears down the veins and can ultimately lead to rupturing and bleeding.
Cerebral AVMs occur in only 0.15% of Americans, but they are one of the most common causes of cerebral hemorrhage in young adults. Most AVMs have an estimated annual hemorrhage rate of 4%; in unique cases that display a more aggressive disease progression, the risk for hemorrhage is much greater (5,6). AVMs can cause headaches, seizures, and stroke-like symptoms. Although all AVMs are difficult to treat, larger AVMs or those present deep within the brain can pose special problems (4). They often require surgeons to undertake a combination of approaches to treatment.
After an AVM has been identified, the first decision that must be made by a physician is whether to treat it. This is a complex decision that takes into account multiple factors, including the patient’s age, medical history, the AVM grade (which is based on size, location, and venous drainage), and whether the AVM has already ruptured. If the physician decides to operate, a treatment strategy is developed. The different surgical strategies currently used to treat AVMs are: microsurgery, radiosurgery, embolization, embolization in conjunction with microsurgery, and embolization in conjunction with radiosurgery.
Treatment for AVMs
Microsurgery
During microsurgery resection, a brain surgeon uses a microscope to delicately separate and remove an AVM from its surrounding brain tissue. The effectiveness of microsurgical AVM resection to prevent hemorrhage and reduce seizures in patients presenting with epilepsy has been established in medical literature (Fig.2) (7,8). Studies have also demonstrated that children undergoing microsurgical resection often fare better than adult patients, although the precise reason why has not yet been explained.
|
|
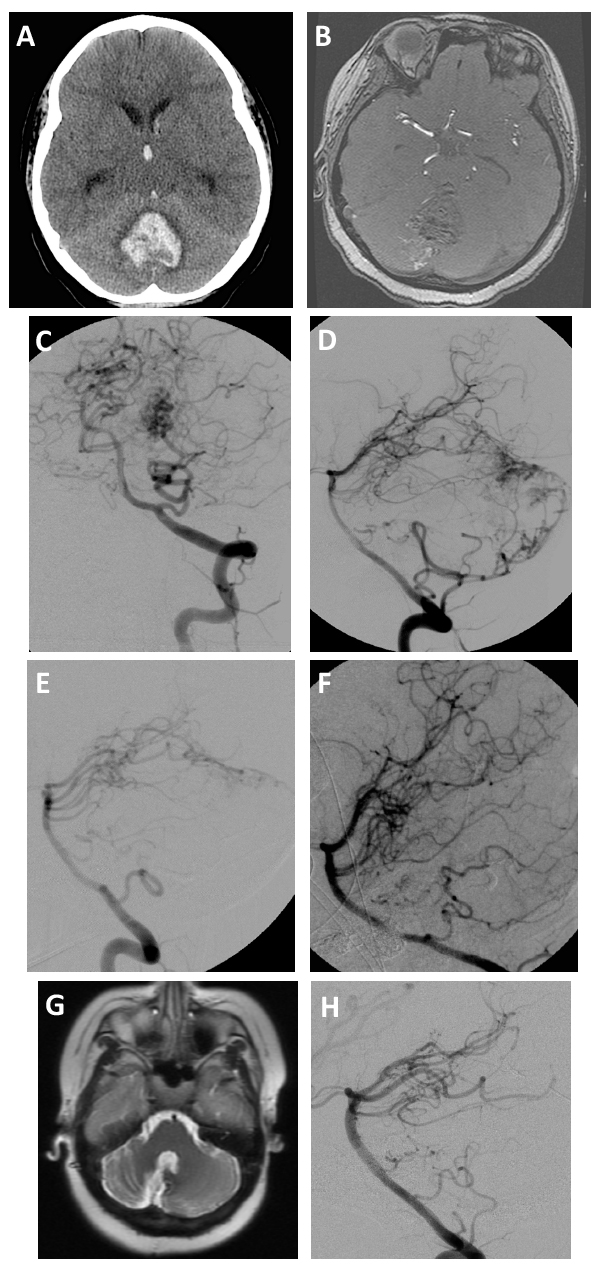
| Figure 2. (A) In this CT scan, the results of hemorrhage are visible in the patient’s posterior fossa with extension into the third ventricle. (B) MRI showed a lesion in the cerebellum. (C–D) DSA depicted the AVM and allowed us to locate the arterial feeders (white arrows). (E–F) Preoperative embolization of the AVM was carried out to optimize the safety and effectiveness of surgical resection. (G–H) Two years after surgery, these follow-up images were taken confirming that the AVM was successfully cured. |
Technological advances in microsurgery over the past three decades have dramatically increased the feasibility of AVM resection, but certain AVMs are associated with unacceptable risks that preclude a microsurgical approach to treatment. In order to calculate a person’s risk for disabling injury or death, physicians use the Spetzler-Martin grading scale, which works by analyzing three specific factors: AVM size, venous drainage pattern, and the proximity of the AVM to areas of the cortex that are crucial for sensory processing, linguistic ability, or motor ability (Table 1) (9).
Table 1Spetzler-Martin Grading Scale* |
|
|---|---|
Grade Feature |
Points |
| Size | |
| Small (< 3 cm) | 1 |
| Medium (3–6 cm) | 2 |
| Large (> 6 cm) | 3 |
| Eloquence of adjacent brain | |
| Non-eloquent | 0 |
| Eloquent | 1 |
| Pattern of venous drainage | |
| Superficial only | 0 |
| Deep | 1 |
| *Grade = {size} + {eloquence} + {venous drainage}; that is {1, 2, or 3} + {0 or 1} + {0 or 1} Reproduced with permission from reference 9. |
|
AVMs that are larger than six centimeters, the presence of deep venous drainage, and a close proximity to crucial cortex areas are all factors that have been associated with unfavorable surgical outcomes (Table 2). If the AVM scores higher than a three on the Spetzler-Martin grading scale, the patient will most likely not be considered a candidate for microsurgery.
Table 2Incidence of postoperative deficit
|
||
|---|---|---|
| Grade | Minor Deficit (%) | Major Deficit (%) |
| I | 0 | 0 |
| II | 5 | 0 |
| III | 12 | 4 |
| IV | 20 | 7 |
| V | 19 | 12 |
| Reproduced with permission from reference 9. | ||
Radiosurgery
Radiosurgery involves the use of stereotactic guidance tools to focus a large dose of radiation onto an AVM. The goal is to irradiate the blood vessels of the nidus so that they thicken and shrink over a period of years, eventually causing the AVM to close or become “obliterated”. The procedure can often be completed in one visit but, in some cases, multiple sessions might be necessary. The main disadvantage of this treatment is that the results are not immediate. During the two to three year period leading up to complete AVM obliteration, the patient is still at risk for hemorrhage.
Radiosurgery is often considered the safest treatment option for patients who are not able to undergo the more invasive microsurgery option due to age, health complications, or the proximity of the AVM to important neural structures. It is an ideal treatment approach for small, deep-seated AVMs (10,11). AVMs with volumes equal to or less than 10 ml have a 78–88% obliteration rate after only three years (12).
Embolization
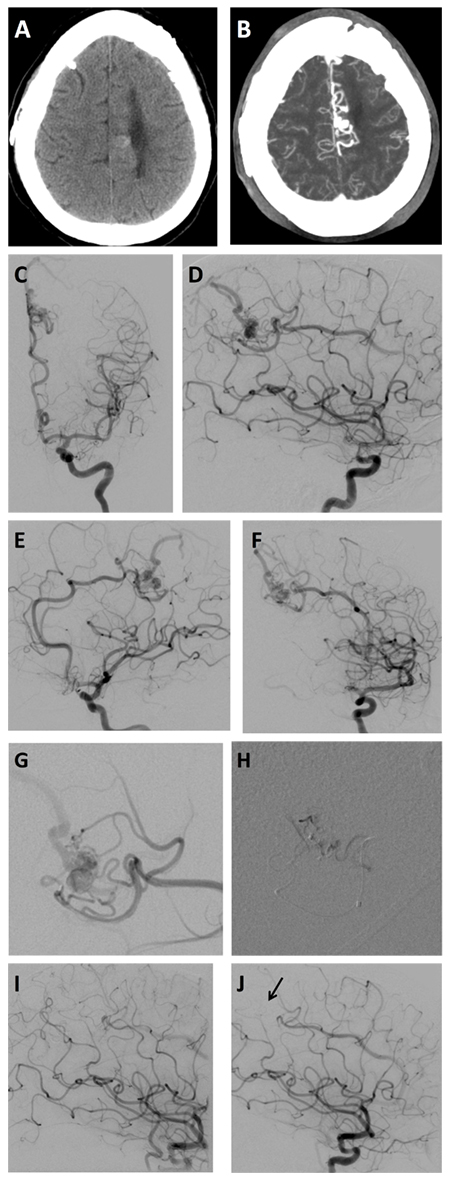 Figure 3. (A) CT scan and (B) MRI revealed a small AVM located near this patient’s midline in the frontoparietal region. (C–F) DSA imaging showed the AVM nidus (white arrows) and draining veins (black arrows). (G) Selective contrast injection depicted arterial feeders and venous drainage of the AVM as well as (H) post-embolization results. (I–J) DSA confirmed complete occlusion of the AVM with embolization agent (black arrow).
Figure 3. (A) CT scan and (B) MRI revealed a small AVM located near this patient’s midline in the frontoparietal region. (C–F) DSA imaging showed the AVM nidus (white arrows) and draining veins (black arrows). (G) Selective contrast injection depicted arterial feeders and venous drainage of the AVM as well as (H) post-embolization results. (I–J) DSA confirmed complete occlusion of the AVM with embolization agent (black arrow).
The goal of embolization is to block off the arteries of the AVM with the highest blood flow and/or the vessels that are the most difficult to manage during surgery (Fig.3). The treatment uses a wire to guide a catheter (small tube) from the femoral artery in the thigh/groin region upward into the brain where the arteries of the AVM are located. The AVM is then injected with materials that restrict the amount of blood that can flow into it.
The procedure is versatile in its application. Its uses vary depending on the treatment strategy developed for each individual patient. Different endovascular AVM embolization strategies include: embolization as a pre-microsurgical tool, embolization as a pre-radiosurgery tool, embolization as the sole curative treatment, embolization for the alleviation of symptoms, and embolization of aneurysms associated with AVM.
One of the reasons why embolization might be implemented as a pre-microsurgical tool is because it reduces blood loss associated with AVM resection by restricting the flow of blood to the AVM (13). Embolized vessels can also serve as a kind of “roadmap” during surgery; they guide the surgeon by defining the anatomical structures of the AVM.Despite these helpful advantages, embolization does carry a risk for bleeding that should also be taken into account before any pre-surgical work is considered.
For radiosurgery candidates, embolization can be used to reduce the volume of the AVM by blocking off its feeding arteries. This is advantageous because AVMs of smaller size have been associated with better radiosurgery success rates (10). In some cases, embolization has been used to reduce precariously large AVMs to sizes that are more amenable to radiosurgery (14,15). A smaller AVM might also permit surgeons to use smaller doses of radiation during treatment.
Endovascular treatment alone is rarely curative (15). However, it might be sufficient for some small AVMs that have a limited number of feeding arteries and draining veins. Under rare circumstances, a medium-sized AVM can be embolized over the course of months until a patient is considered cured.
If AVMs are too dangerous to remove because of their size and/or location, embolization might be employed solely to alleviate a patient’s symptoms. Because AVMs do not have capillaries, they might “steal” blood from the surrounding tissue. This “theft” deprives the surrounding tissue of oxygen and vital nutrients, which can bring about debilitating symptoms for AVM patients, such as severe headaches. Embolization can help by relieving the severity of these problematic symptoms.
Embolization can also be used to treat aneurysms associated with AVMs. Aneurysms are weak, bulging areas in the wall of an artery that are at a risk for rupture. They compress the surrounding tissue and potentially cause nerve paralysis, headaches, neck pain, upper back pain, nausea, and vomiting. If an aneurysm ruptures, the subsequent bleeding can result in stroke or even death. The severity of these consequences motivates surgeons to treat aneurysms first before addressing AVM treatment. By embolizing the aneurysm, the surgeon blocks the flow of blood and prevents the arterial bulge from rupturing, which makes any subsequent surgical intervention safer and more effective.
References
- Ondra SL, Troupp H, George ED, Schwab K. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg. 1990;73:387-391.
- Stein BM, Wolpert SM. Arteriovenous malformations of the brain. I: Current concepts and treatment. Arch Neurol. 1980;37:1-5.
- Rothbart D, Awad IA, Lee J, et al. Expression of angiogenic factors and structural proteins in central nervous system vascular malformations. Neurosurgery. 1996;38:915-925.
- Wallace RC, Flom RA, Khayata MH, et al. The safety and effectiveness of brain arteriovenous malformation embolization using acrylic and particles: the experiences of a single institution. Neurosurgery. 1995;37:606-618.
- Thompson RC, Steinberg GK, Levy RP, Marks MP. The management of patients with arteriovenous malformations and associated intracranial aneurysms. Neurosurgery. 1998;43:202-212.
- Mansmann U, Meisel J, Brock M, et al Factors associated with intracranial hemorrhage in cases of cerebral arteriovenous malformation. Neurosurgery. 2000;46:272-281.
- Heros RC, Korosue K, Diebold PM. Surgical excision of cerebral arteriovenous malformations: late results. Neurosurgery. 1990;26:570-578.
- Heros RC, Morcos J, Korosue K. Arteriovenous malformations of the brain. Surgical management. Clin Neurosurg. 1993;40:139-173.
- Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476-483.
- Flickinger JC, Pollock BE, Kondziolka D, Lunsford LD. A dose-response analysis of arteriovenous malformation obliteration after radiosurgery. Int J Radiat Oncol Biol Phys. 1996;36:873-879.
- Kondziolka D, Lunsford LD, Kanal E, Talagala L. Stereotactic magnetic resonance angiography for targeting in arteriovenous malformation radiosurgery. Neurosurgery. 1994;35:585-591.
- Pollock BE, Lunsford LD, Flickinger JC, Kondziolka D. The role of embolization in combination with stereotactic radiosurgery in the management of pial and dural arteriovenous malformations. In: Connors JJ, Wojak JC, eds. Interventional Neuroradiology. Philadelphia: W.B. Saunders; 1999:267-275.
- Jafar JJ, Davis AJ, Berenstein A, et al. The effect of embolization with N-butyl cyanoacrylate prior to surgical resection of cerebral arteriovenous malformations. J Neurosurg. 1993;78:60-69.
- Gobin YP, Laurent A, Merienne L, Schlienger M, Aymard A, Houdart E, Casasco A, Lefkopoulos D, George B, Merland JJ. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg. 1996;85:19-28.
- Wikholm G, Lundqvist C, Svendsen P. The Goteberg cohort of embolized cerebral arteriovenous malformations: a 6-year follow-up. Neurosurgery. 2001;49:799-806.
- Batjer HH, Purdy PD, Giller CA, Samson DS. Evidence of redistribution of cerebral blood flow during treatment for an intracranial arteriovenous malformation. Neurosurgery. 1989;25:599-605.
- Mohr JP, Parides MK, Stapf C, et al. for the international ARUBA Investigators. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383:614-621.
- Al-Shahi SR, White PM, Counsell CE, et al. for the Scottish Audit of Intracranial Vascular Malformations Collaborators. Outcome After Conservative Management or Intervention for Unruptured Brain Ateriovenous Malformations. JAMA. 2014;311(16):1661-1669.
- Van Beijnum J, van der Worp HB, Buis DR, et al. Treatment of Brain Arteriovenous Malformations A Systematic Review and Meta-analysis. JAMA. 2011;306(18).2011-2019.